FDA Approves Pfizer Vaccine for Children Aged 12 and Up
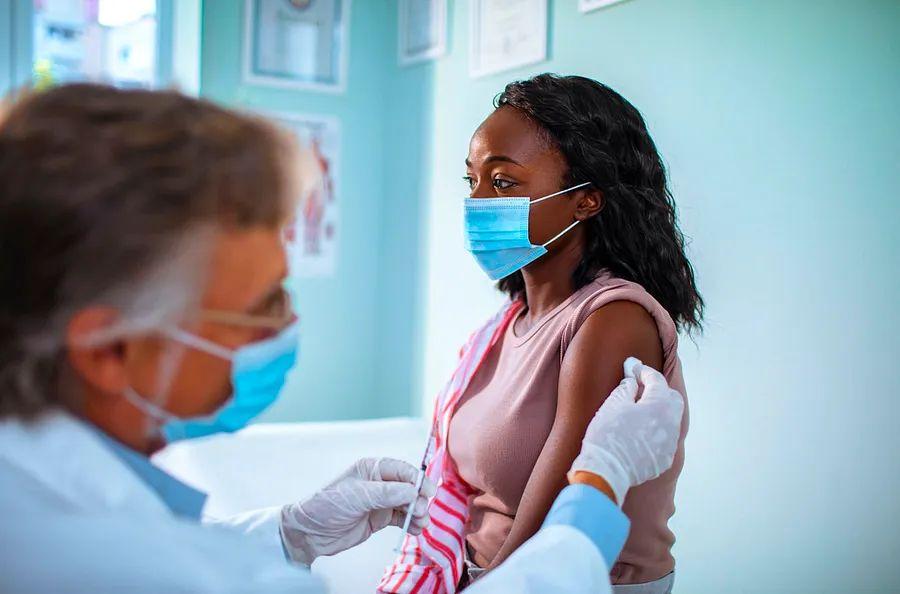
In a significant development for travel, the Food and Drug Administration has authorized the Pfizer COVID-19 vaccine for children between the ages of 12 and 15 in the United States.
This is a vital advancement in the battle against the pandemic and could encourage many families to make travel plans. Numerous parents have been waiting for news on vaccinations for kids before booking family vacations. However, this isn’t the last step before the vaccinations can commence for that age group. The Centers for Disease Control needs to convene to review the data and provide approval. That meeting may occur in the next few days, enabling vaccinations to start immediately afterward.
In a statement, the agency remarked, "The FDA has concluded that the Pfizer-BioNTech COVID-19 Vaccine fulfills the necessary criteria to amend the Emergency Use Authorization (EUA), and the known and potential advantages of this vaccine for individuals 12 years and older surpass the known and potential risks, thereby endorsing its use."
William Gruber, Pfizer's Senior VP of Vaccine and Clinical Research and Development, mentioned in an interview with Bloomberg that "Children might be the 'remaining engine fueling the pandemic.' It's crucial for us to address that issue."
Research on vaccine use for children under 12 is currently in progress, with potential approval expected by the end of the year.
The Pfizer-BioNTech vaccine received approval for adults in the U.S. following an emergency-use authorization from the Food and Drug Administration (FDA) in December. It marked the first vaccine granted approval for adults in the United States.
Image credit: Marko Geber/Getty Images.

1

2

3

4

5
Evaluation :
5/5